TOXIC LOTS OF SARS-CoV-2 JABS WERE ALWAYS GOING TO HAPPEN
You can't shortcut on quality in the supply chain
[First posted Feb 08, 2022]
WHAT TOXIC LOTS (BATCHES)?
December 29, 2021, Senator Ron Johnson wrote to Janet Woodcock, M.D. Acting Commissioner Food and Drug Administration and Rochelle P. Walensky, M.D., MPH Director Centers for Disease Control and Prevention.
The letter began:
Due to the unprecedented number of adverse events and deaths associated with the COVID-19 vaccines on the Vaccine Adverse Event Reporting System (VAERS), independent researchers have downloaded VAERS data and begun analyzing the apparent variation in the distribution of adverse events between vaccine lots. If the production of vaccines were under control, with quality systems working properly, one would expect to see relatively even distribution of adverse events and deaths across all lots.
According to these researchers, the variation of adverse events among COVID-19 vaccine lots stands in stark contrast to a much lower degree of variation of adverse events associated with seasonal flu vaccine lots reported over a 30-year period.
The entire letter can be viewed here, and it is concerning to say the least. Senator Johnson asks detailed and searching questions about what appears to be going on, and wrong, in the supply chains for the jabs.
To date, answers have been slow to emerge, and it’s not surprising.
WHY NO SURPRISE?
The regulatory authorities (FDA/MHRA/EMA) are charged with checking out every detail of the supply chain, leaving no stone unturned. They must critically review the data provided by the company intending to sell the product of the supply chain—it will go into people’s bodies, so it must be squeaky clean.
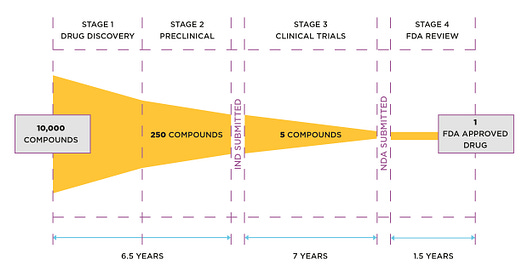
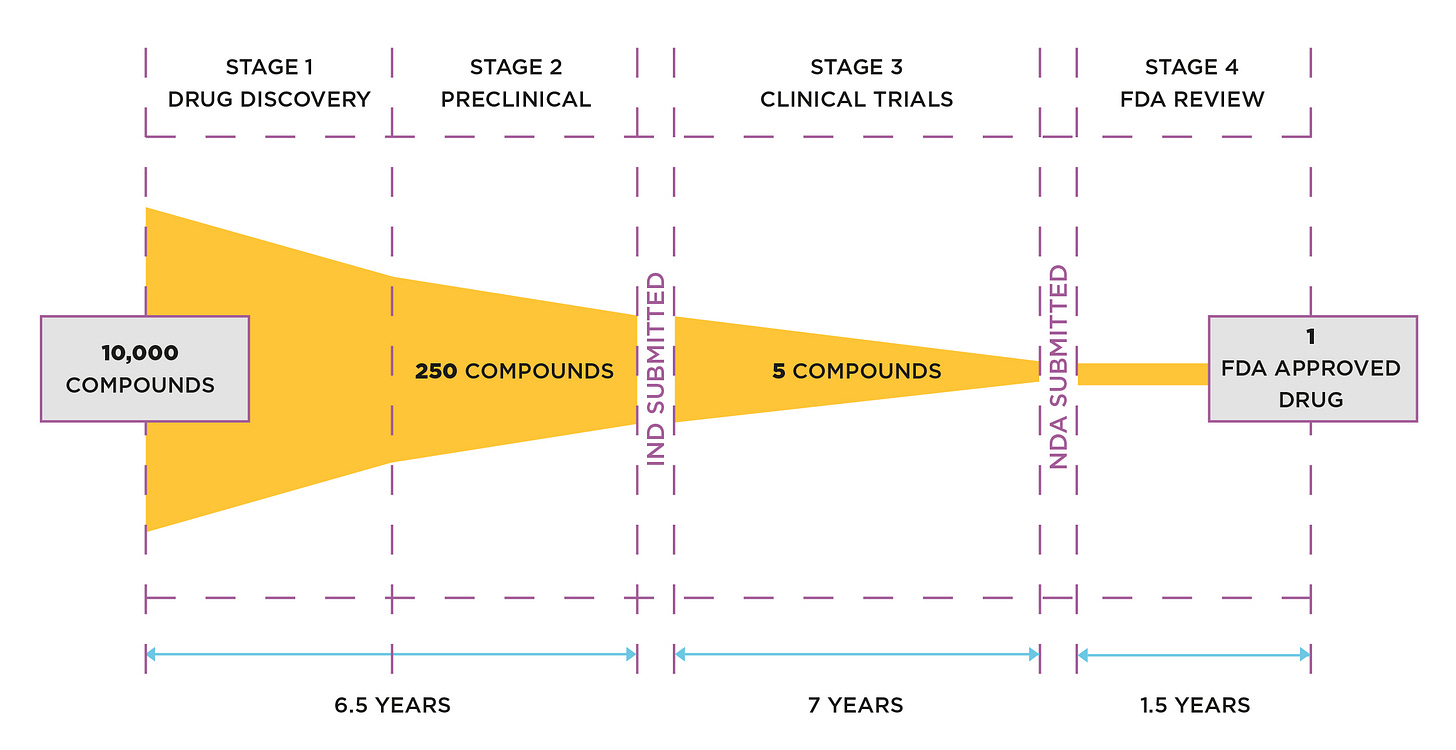
The supply chain portion of review by regulators is known as the chemistry, manufacturing & controls (CMC) section. There are two other sections, containing safety and clinical data. These are submitted and reviewed as a single document known as the electronic common technical document (eCTD).
The right hand side of the diagram below shows the FDA review of the eCTD—typically 1.5 years. It takes that long because each section impacts the other. For example, a change in the supply chain can impact product safety and its clinical effect.
The ‘rolling reviews’ for the SARS-CoV-2 injections took just a few months; and it has now emerged, thanks to the work of our collaborators, that the regulatory reviews were of R&D lots that could not have been produced to the required standard.
In effect, given such a cursory regulatory review of the supply chain, toxic lots were guaranteed to occur—if you cut corners with any physical supply chain, things will go wrong, the only unknown is exactly how, where, and when.
WHAT SHOULD HAPPEN NEXT?
There is more than enough evidence to justify a complete hold on all manufacture of injections, and a thorough investigation carried out, with remediation based on findings. That would be the prudent approach.
Only Governments have the power to do that.
Ergo, we should all get behind Senator Ron Johnson (and his independent researchers), Senator Rand Paul, and others in US Senate working to inject clarity into big pharma’s murky creation.
There is no alternative option.



The variations in toxicity per batch confirms the manufacturers are not complying with cGMP or I. per the EUAs for Pfizer and Moderna.
Toxic drugs are always happening. They can be nothing else and your body does not like them.