COVID SUPPLY CHAINS: FACT NOT FICTION—CHAPTER 3
The truth, the whole truth, and nothing but the truth
Now we get into gene therapy…
This Chapter 3 of THE COVID-19 SUPPLY CHAIN: Fact not Fiction is where we turn to the specifics of gene therapy, and its fatal flaws:
CHAPTER 3 PHARMACEUTICAL SUPPLY CHAINS
The Pharmaceutical Distribution System
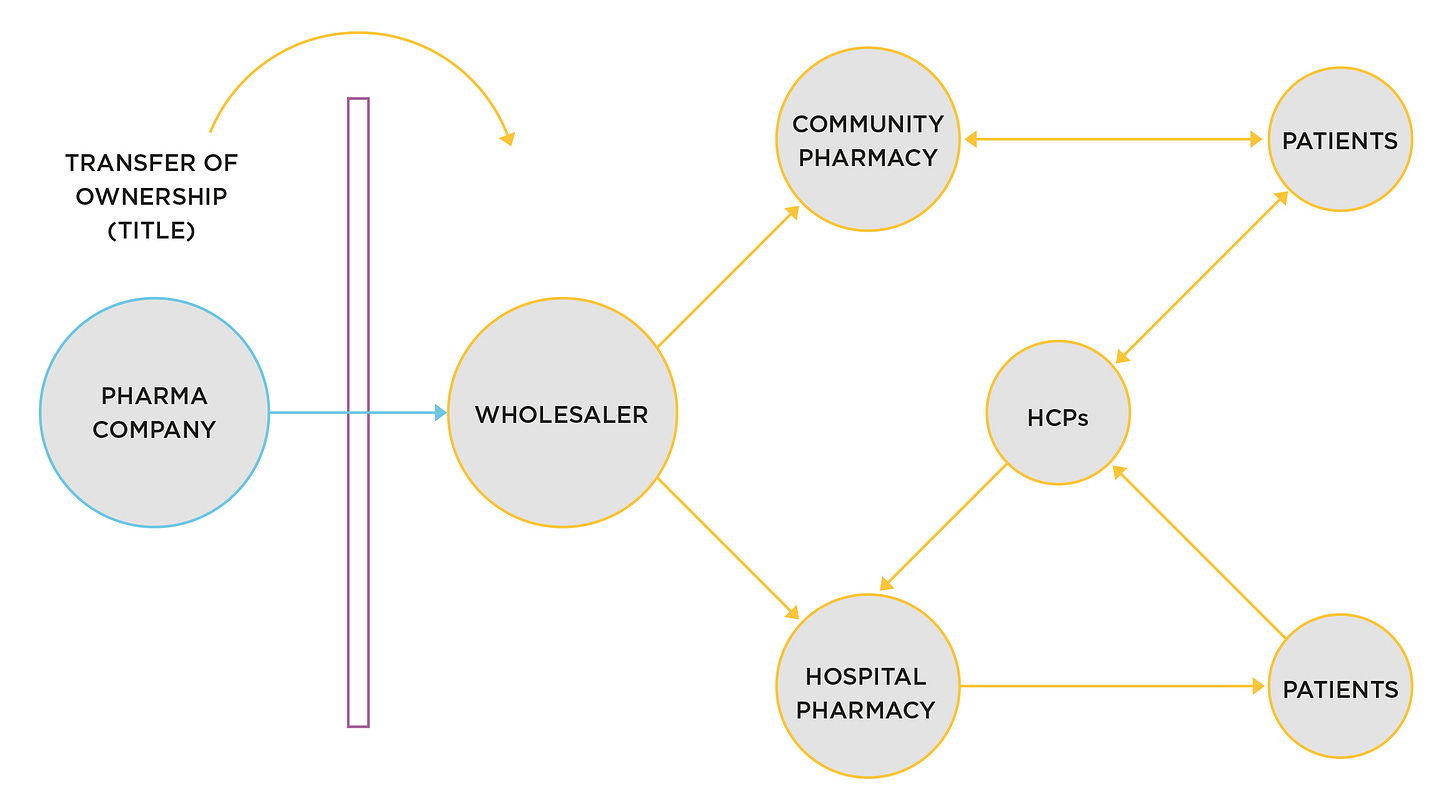
Once produced and approved for sale, both small molecule and biologic drugs share the same distribution channel to market. Figure 1 shows a simple diagram.
Figure 1. Stages in the distribution supply-chain for small and large-molecule (biologic) products
Both small-molecule and biologic products move through the distribution supply-chain. The wholesaler stores and moves finished products to pharmacies to be available for patient use. Wholesalers are regulated in the same way manufacturers are, under Good Distribution Practice regulations (EU), or equivalent in countries outside the EU/UK.
Wholesalers buy the products from the companies holding the marketing authorisations (aka product licenses). From that point onwards, the license holders are no longer involved in the physical distribution, although they still have responsibility for pharmacovigilance (collecting reports of adverse events and acting on them where relevant).