FDA unearths a minefield of quality issues at the contractor manufacturing Moderna's jabs
That’s a product recall if ever there was one...
[Note: This is a recycle from 2022]
The Achilles' heel of mRNA jabs
The Achilles' heel of these mRNA jabs has always been the manufacturing and distribution supply chain. Most people don’t realise it, because they have been conditioned to believe drugs are found by accident, not developed and produced like other consumer products.
In developing and manufacturing the jabs 10x faster than ever before, multiple c$ck-ups were going to occur. This is the latest we know about, because US FDA has gone in and done the inspection:
Moderna's new booster launch tripped up by production issues at Catalent plant, by Kevin Dunleavy, FiercePharma, Sep 21, 2022 11:23am.
At last, we are seeing the dreaded and much feared (pre-pandemic) FDA 483 Form being issued. This is it below, taken from the article:
FDA 483 Form Catalent Bloomingdale
It contains substantial findings that could leave a person wondering if that plant can go on operating without major corrective actions—that would take months if not a year or two, as these are deep rooted issues. These are the first four Observations made:
OBSERVATION 1
Your firm failed to thoroughly investigate any unexplained discrepancy or failure of a batch or any of its components to meet any of its specifications, whether or not the batch has already been distributed.
OBSERVATION 2
Written records of an investigation of drug complaint do not include the findings of the investigation and the follow-up
OBSERVATION 3
Control procedures are not established which monitor the output and validate the performance of those manufacturing processes that may be responsible for causing variability in characteristics of the in-process material and the drug product.
OBSERVATION 4
Acceptance criteria for the sampling and testing conducted by the quality control unit is not adequate to ensure that batches of the drug product meet appropriate statistical quality control criteria as a condition for their approval and release.
The remaining OBSERVATIONS 5 - 12 can be viewed on the link above or here.
It makes chilling reading if you have even the slightest idea of how errors in the supply chain can impact product safety.
Why is this great news?
At last, a Regulatory Authority has honoured its right to physically inspect a key contractor in the mRNA supply chain. Far too much has been left to virtual inspections up until now, which are worse than useless for getting to the root of issues on site.
If this expands to Lonza, the contractor manufacturer for the Moderna drug substance (active pharmaceutical ingredient), the whole sorry mess will be exposed. No oversight from Pfizer, a catalogue of non-compliances, patient safety issues at every stage in the end-to-end supply chain.
That’s a product recall if ever there was one—but is Moderna geared up to do one?
Moderna not geared up to do a product recall
If there is a recall, it will be an immense undertaking. Five months ago, I posted on a previous issue with another Moderna contractor (!), and the likelihood Moderna could execute a product recall:
This is an extract:
It's Moderna’s responsibility to get it done
The responsibility is wholly on Moderna to get this done, as part of their legal obligations to manage the entire supply chain, end-to-end, including distribution to vaccination centres for patient administration.
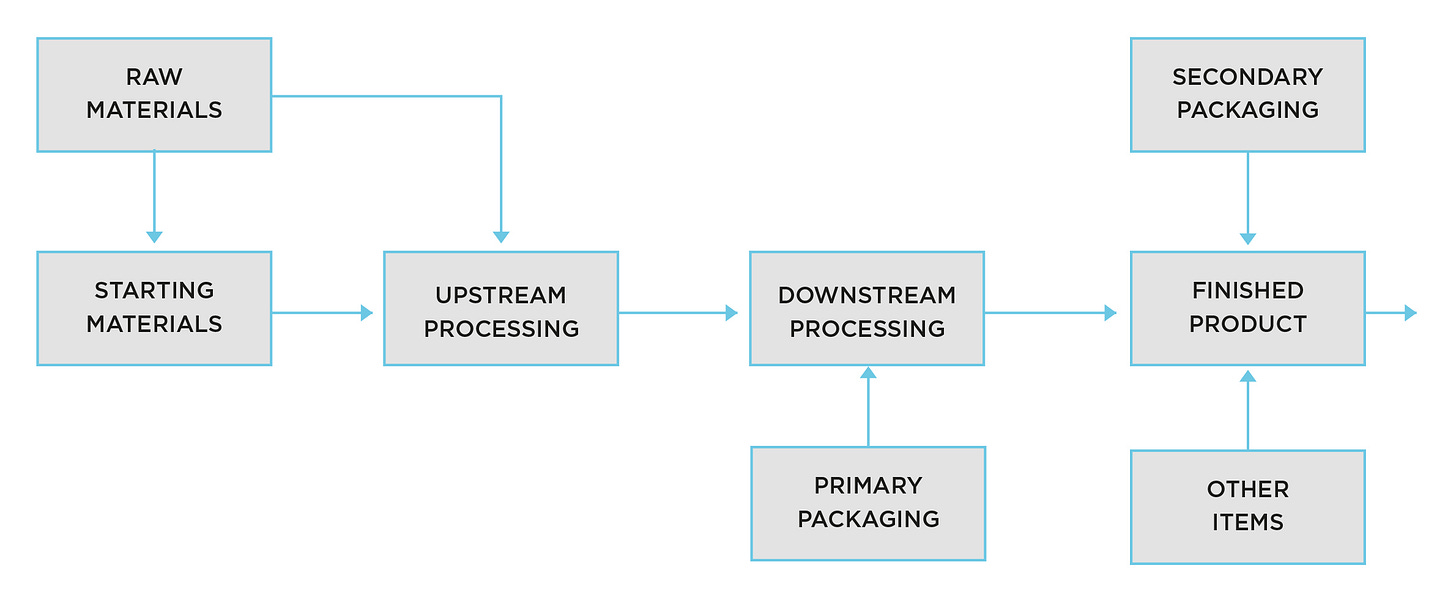
The block diagram below demonstrates the extent of any recall. It may look simple, but remember, in some blocks there are 10s, even 100s of different suppliers.
The issue appears to be in the block labelled DOWNSTREAM PROCESSING, where drug substance in filled into vials, a bung inserted and an aluminium seal applied.
Rovi staff are likely to be running around like headless chickens, wondering what has gone wrong, so they can pass the news on to Moderna…but do they know what vials went where?
Likely not...since there is another box before the vials go to the vaccination centres, FINISHED PRODUCT.
Normally, in the olden days, pre-regulatory capture, that means packaging the product before it goes to the wholesalers, as a unit dose, in a carton with a patient information leaflet inserted. Once the wholesalers have it, they work to the good practices they are bound by under pharmaceutical law.
Since these injections were/are frozen down to -20 degrees C, the wholesalers did not have the cold chain capabilities to handle them. So, who did?
Also, what company did the freezing operation on the vials in trays?
Moderna must know all this in order to carry out a recall.
Moderna should already have run a mock recall to ensure it all works properly ahead of a situation like this.
Then there are all the upstream suppliers involved, that could be part of the problem.
Hmmm…not sounding good, eh???
FDA as game changer 🧨💥
If FDA keeps up this excellent move towards unearthing the truth lurking in the mRNA supply chains, it will be a game changer, as you can't deny the power of real-world evidence collected by real-world expert inspectors.
My question to whom it may concern is this: US FDA has the discretionary power, under the code of federal regulations (CFR) Title 21, to close down plants where an inspection reveals systemic failure throughout an Establishment.





over at TTE, (Trust the Evidence) with Carl Heneghan and Tom Jefferson; they are talking of how minutes put up by MHRA were rapidly pulled down; https://trusttheevidence.substack.com/p/the-mhra-papers-comparison-of-the
poor old MHRA; they never thought that TTE would have downloaded all the files as they were put up; so they have reviewed the changes; it all seems to be
"A comparison of the two sets of files shows 60 changes with three replacements, 17 insertions, and 40 deletions. What follows are the most significant parts of the old geezer’s views."
"Note that “batch” occurs 202 times in the redacted file and 243 times in the unredacted file. The primary purpose of these files was to remove any mention of the manufacturing process and the batch identification and content.
We welcome your thoughts on whether the information is commercially confidential or in the public interest of being made available so we can better understand the safety issues."
So presumably the bosses of the MHRA wanted all mentions of manufacturing process removed; well, if they pay 86% of their wages, they are entitled to do that, aren't they?
Tom Jefferson did another post soon after the above; where he tries to make sense of minutes being put up; then rapidly pulled down; they put up again; what were they trying to hide some suspicious minds mused?
Tom in this second piece echoes what Hedley has talked of extensively here; that folks need to know how stuff is made; and what is in it; that is potentially being injected into them
https://trusttheevidence.substack.com/p/commercial-in-confidence-screen-or
If the FDA was doing its job in the beginning of the fake covid pandemic, none of these poison mRNA injections would have ever hit the beaches. And millions of people would still be alive and many millions more would not have suffered debilitating reactions. No excuse possible or accepted.
Sorry, too little too late and I couldn't care less if the FDA or CDC even survive another day.