Is UK MHRA playing with 'advanced therapy' fire, by allowing hospital pharmacies to complete final stage manufacture?
Yes, absolutely
Advanced Therapy Treatment Centers (ATTCs)
In the UK, “Advanced Therapy Treatment Centres” have been established as part of an ongoing Government initiative. Its mission is explained in the website, as follows:
“Our Mission
The ATTC project aims to develop robust systems for the routine delivery of ATMPs as a standard of care throughout the NHS in the United Kingdom.”
It then goes on to say:
“Over the last five years, new cell and gene therapies have been developed to treat some cancers and inherited diseases. These advanced therapies are different from existing treatments in two important ways: they are designed to restore normal function, sometimes offering cures where an unmet medical need exists and they require new ways of working by the NHS.”
About Advanced Therapy Medicinal Products (ATMPs) in EU/UK
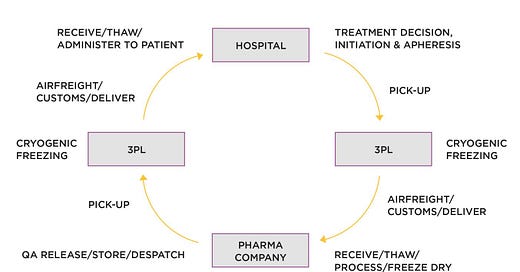
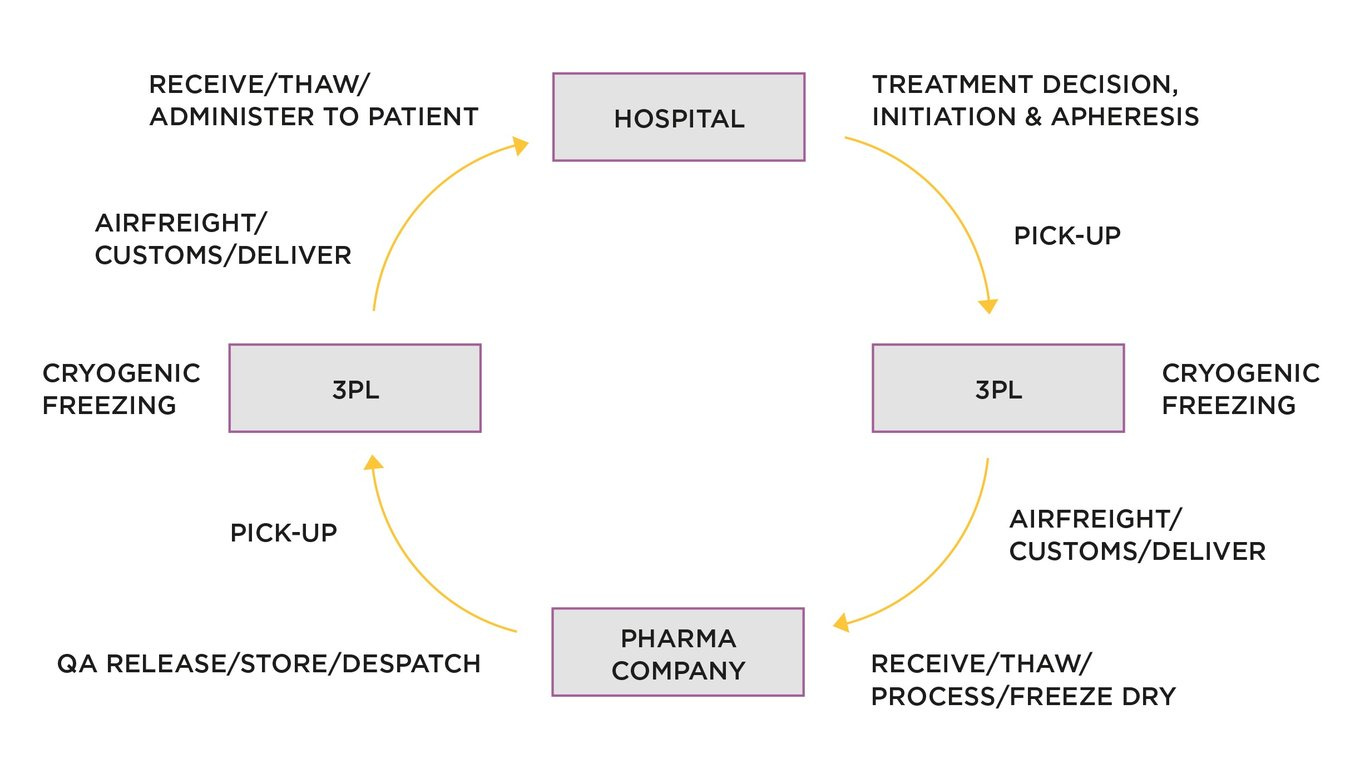
We know that within the EU/UK definition of Advanced Therapy Medicinal Products (ATMPs), there lay somatic-cell therapy, gene therapy and tissue engineered products. Additionally, there is a split between autologous (patient specific) and allogeneic (from a donor population) therapies.
We also know that by far the most commercially advanced ATMP is CAR T treatments for rare blood cancers, such as Novartis' Kymriah, approved by FDA August 2017. However, companies developing and selling CAR T products are still wrestling with the massive supply chain challenges brought about by the autologous nature of CAR T—the patient is in a hospital bed, and manufacture takes place hundreds of miles away, in a manufacturing plant.
In this previous post, working with TrialSite founder and CEO, Daniel O'Connor, the issues were explained:
Gene therapy—is it really a sound investment prospect?
We concluded this: