Pharma product development creates a spaghetti—and patients can die
...that's at normal speed—what about warp speed?
Introducing the spaghetti
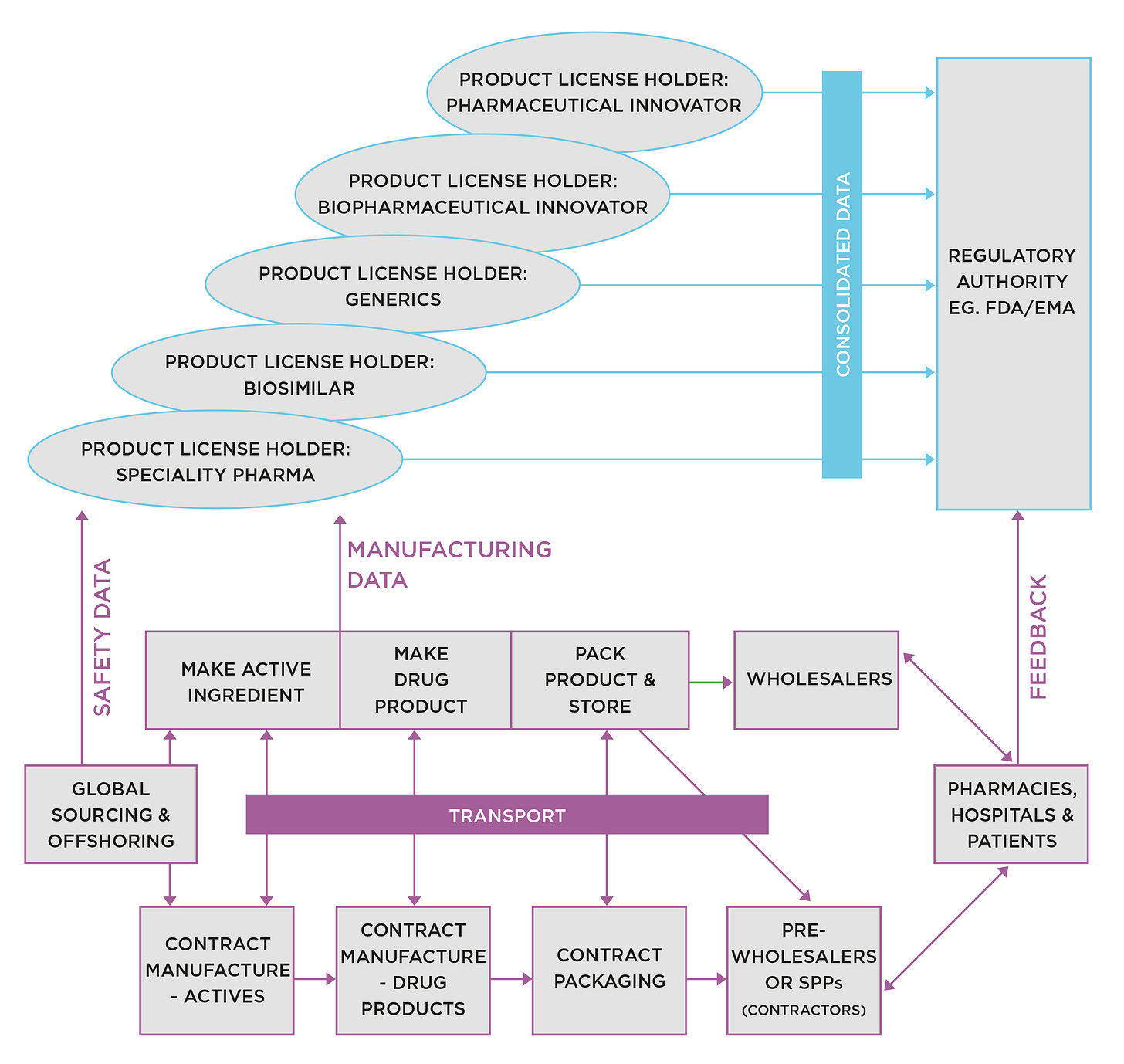
The bottom half of Figure 1 shows the spaghetti supply chains that emerge from the pharmaceutical product development process.
With my consulting hat on, there seems to be little awareness among actors in the development process of the seminal impact decisions taken there can have on the quality, cost, and service performance of the future commercial supply chain.
Focus is exclusively on producing the data required to gain regulatory approval. The consequences can be debilitating for the future supply chain. Here are some of the problems that may have been locked-in during development:
Scarce/bespoke materials specified
Sole sourcing from far off countries
Inappropriate dosage form selected
Contractors with insufficient capacity or capability
Poor process yields
Weak compliance with agreements
Analytical testing methods not accurate
Shipping/storage conditions not adequately defined
Incorrect value declarations to customs
Weak, hands length contractor relationships
With my consulting hat on again, the complexity of development supply chains when they are mapped out never ceases to amaze me. It is hard to find any kind of logic or strategy driving decision-making.
For example, modern-day thinking is that companies benefit from working closely with their suppliers of key components and materials. This is especially the case when there are multiple production stages to be carried out.
I see little evidence of that happening in pharmaceutical drug development. The crucial point missed is that the supply chain data (shown in Figure 1), once submitted to the Regulatory Authority for review, cannot change before launch. It is also likely it will never, ever change, as it is so expensive and disruptive to do so.
That means material and process specifications, test methods, suppliers, producers, service providers, or anything else, are locked in for life. It is, therefore, imperative to get it right before the filing goes in.
Unfortunately, all too often, the focus is on data collection, especially clinical efficacy. It is as if the prime purpose of a supply chain—to deliver fit-for-purpose products and services to customers—has been overlooked.
The result is an almost infinite set of permutations for the physical flow of materials and products. Each hand-over represents an opportunity for error that could be done without.
The potential for patient harm—the heparin tragedy
It is not just issues locked into the commercial supply chain at launch. There is potential for a far more serious outcome.