What Patients Need to Know about Pharmaceutical Supply Chains—Chapters 5 & 6
Everything a critcal thinker needs to know about the supply chains for SARS-CoV-2 injections—packed with facts and evidence that can't be refuted.
[Note from Hedley: you may not have noticed, but yesterday’s email was a draft that was not quite finished, which I sent in error. Here it is finished, along with Chapter 6; please note also the final sentence—”ponder what could have gone on when SARS-CoV-2 injections were developed “at the speed of science”?”]
5. PRODUCT DEVELOPMENT AND SUPPLY CHAINS
Pharma doesn’t follow other industries.
In most industries, supply chains are created as part of a new product development program. The developers work together, consulting product end-users, producers, and distributors to ensure the physical supply chain they put together can deliver what is required by consumers.
For reasons we shall go into later, pharmaceutical new product development does not follow the customary approach of other industries. Rather than beginning with a consumer and working upstream to the beginning, the process begins with a patented compound and moves forwards.
This results in supply chains ‘evolving’ with the passage of time, rather being designed and planned with the consumer in mind. This is how it works, taking the most straight forward example of small molecule products.
The ‘R’ of R&D (Discovery Research) discovers (or finds) molecules using advanced technologies such as molecular modelling. Once it is confirmed a patent is in place, promising compounds are handed to ‘D’ (Development).
Development is a completely new team of specialists responsible for building a supply chain for preclinical testing initially. That means proving the test compound that will be produced by the supply chain is safe to study in humans.
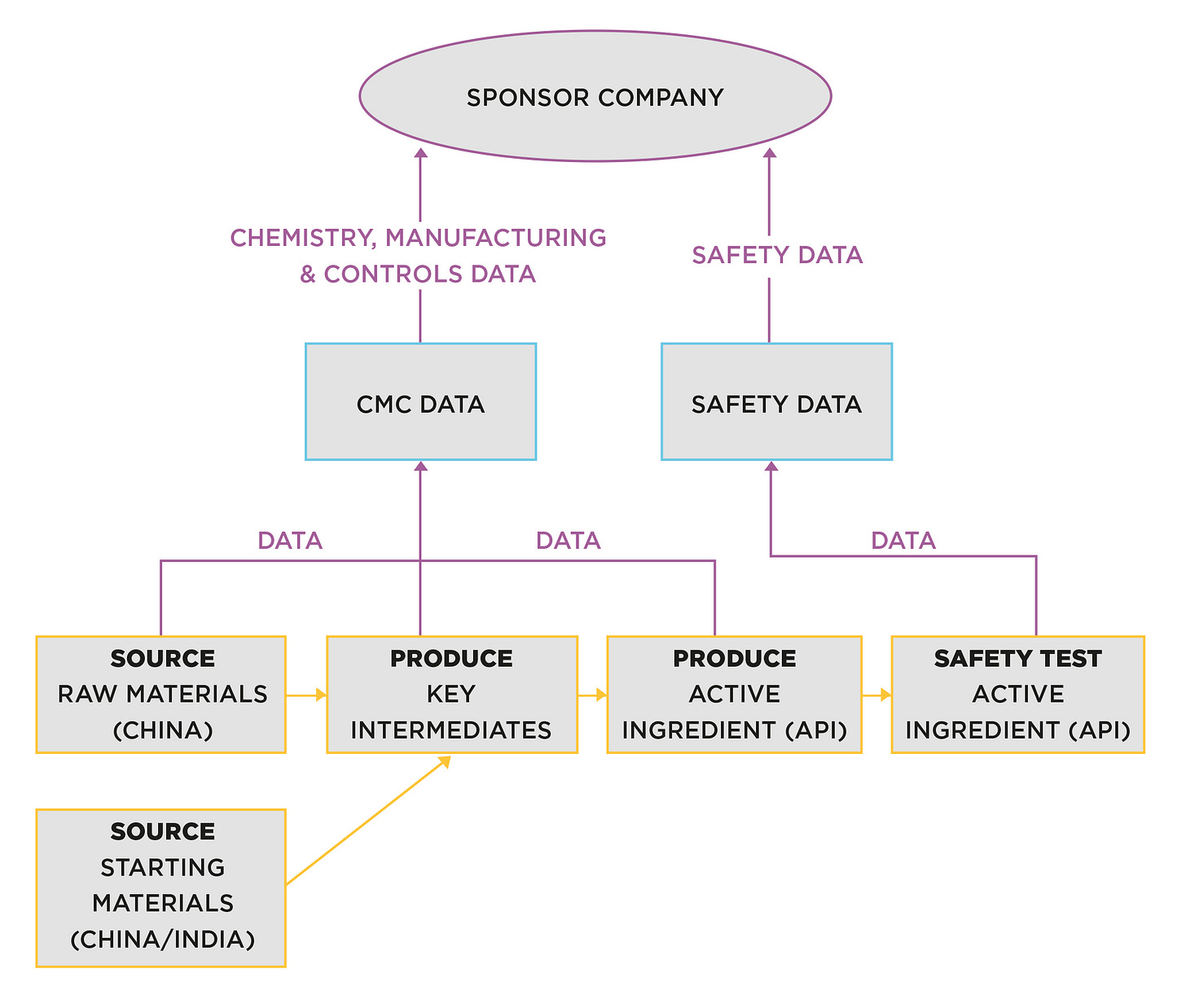
Supply chain for preclinical testing
For small molecule products, around 5 – 10 kilograms of compound will be produced. Figure 1 shows a typical small molecule supply chain:
Figure 1. Production preclinical supply chain
The production process is devised by a ‘route scout’ (process chemist). Their job is to convert a laboratory process that had only ever produced small gram quantities, into a process that can yield much larger quantities.
The skill involved is about making the process as efficient and ‘manufacturable’ as possible. A small-scale pilot plant is normally used at this stage, as the volume is not sufficient to justify occupation of commercial-scale plant.
Figure 1 shows the production of the active pharmaceutical ingredient (API).
Sources of raw and starting materials will be selected by the scientists working on the project. In this case, there are intermediate chemicals involved. Intermediate stages are not always required; it depends on the chosen route of synthesis.
Once produced, the API is transported for safety testing at one or more chosen CROs [Contract Research Organisations]. Even though this appears to be a simple supply chain, there is already an array of suppliers and service providers involved, often spanning the globe.
Remember, raw, starting materials and intermediates are sourced primarily from China and India, so ex-Asian countries are operating a long way from home. For Western companies, this puts a limit on due diligence in selecting sources, and oversight when selected sites are in operation. It also adds significantly to lead-time and complexity.
If it looks like the results support moving to studies in humans, the company will fill out and submit an IND. The IND must include all the details about the supply chain and safety data. If approved by the RA, the company will be awarded an IND and is designated a clinical trial sponsor (CTS).
The CTS will plan to produce product for the first stage of clinical trials - phase I studies in healthy volunteers. The compound being studied is known as ‘test material’.
This is the beginning of an application to market the product, known as an NDA {New Drug Application] or BLA [Biologics License Application].
The programme of work that needs to be carried out by the CTS to prove quality, safety and efficacy is laid down by the RA in the Common Technical Document (eCTD). The details of the end-to-end supply chain have to be specified in Module 3 of the eCTD.