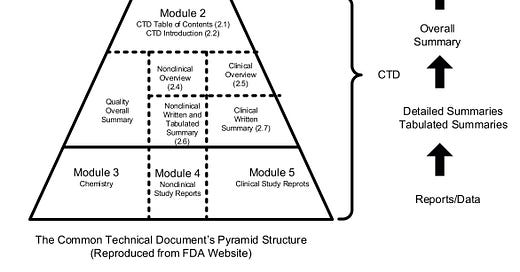
WHAT’S STOPPING DOCTORS REPURPOSING GENERIC MEDICINES? ANSWER—NOT A LOT!
If only they knew this during SARS-CoV-2
[Note: This article appeared in GMP Review AUG/SEPT 2021 when Hedley was helping Dr Tess Lawrie as Director, New Product Development & Supply, EbMCsquared CiC. This is recycled from January 2022]
WHAT’S STOPPING DOCTORS REPURPOSING GENERIC MEDICINES?
For decades, it has been assumed only pharmaceutical companies have the wherewithal to develop or repurpose medicines. This article challenges that assumption based on changing business models in the industry. These changes have created a skills-base of qualified contractors and contract organisations able to offer everything required to bring a medicine to market.
The message to doctors, other health professionals and healthcare systems (referred to as doctors from here on) is that there is just one gap in their armoury—knowledge of the supply-chain that delivers medicines to patients.
COULD DOCTORS REALLY DO IT THEMSELVES?
SARS-CoV-2 has made a case for doctors having access to repurposed generic medicines when they are found to work in me…